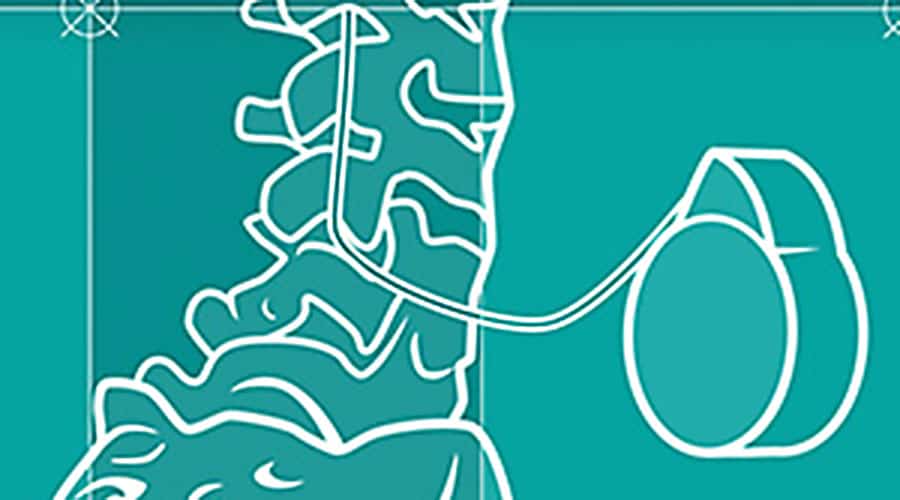
Intrathecal drug delivery is an underused option that merits consideration.
Among patients with cancer, pain associated with the disease and its treatment is often more feared than cancer death itself. Between 65% and 85% of oncology patients experience significant pain at some point in the disease progression, with 40% to 89% experiencing severe pain every day.1,2
As cancer treatment options continue to improve, patients are living longer with lingering pain. Pain tolerance often decreases with repeated exposure, and pain threshold may be decreased by symptoms such as fatigue, anger, apprehension and sleep deprivation.3-5 Patients have stated the importance of symptom management over any other need.6 The undertreatment of cancer pain indicates that the current standard of practice is inadequate in drug type or potency.1,2,7,8
Primary care providers are commonly contacted by oncology patients for help with pain control, but many clinicians provide inadequate pain medication or give “just enough” to tide patients over.7 This is mainly due to a lack of knowledge about pharmacologic therapy in cancer pain.7 Despite the documented safety and efficacy of interventional pain management techniques, these options are rarely explored.9 In 2008, the International Association for the Study of Pain declared a Global Year Against Cancer Pain to encourage the development of cancer pain management activities worldwide.10 This article seeks to raise awareness about those activities by outlining the need for adequate cancer pain management and discussing intrathecal drug delivery as an option for oncology pain.
Clinical Presentation
Three common causes of oncology pain are disease advancement, treatment modalities and previously existing co-morbidities.5,11,12 Infection and inflammation, nerve pressure from tumor presence or enlargement, tissue damage, stretching of visceral surfaces, or obstruction of ducts and intestine are typical mechanisms responsible for oncologic pain.5,13,14 A majority of oncology patients present a mixed pain type of nociceptive and neuropathic pain.10,15 Neuropathic pain is difficult to treat and can be severe in cancer patients.8 Fortunately, a smaller percentage of patients have purely neuropathic pain. Deafferentation, a type of neuropathic pain classified as a permanent loss of sensory input from any part of the body, may result from tumor infiltration of nerve tissue or damage secondary to radiation, chemotherapy or surgical resection of a nerve. Hyperactivity of the somatosensory thalamus and cerebral cortex results in a constant, dull ache mixed with burning and/or shock-like pain that responds poorly to many analgesics.5,14
Inflammation is commonly associated with cancer and may result from tissue damage secondary to treatment modalities. The widespread effects of inflammation lower the threshold for nociceptive depolarization throughout the body.14
Under normal circumstances, increased sensitivity to pain resides with healing. When primary afferent function is altered by injury or disease of the nervous system, hyperalgesia may persist and be resistant to treatment.5
Interventional Management
 Advanced methods of cancer pain management, such as an implanted intrathecal drug delivery system (IDDS; i.e., SynchroMed), should be explored in certain circumstances. These circumstances include when systemic drugs fail to control moderate to severe pain; when adverse effects are intolerable; when neuropathic pain is present; when neuroablation has failed; in anticipation of symptom management; or upon patient request.9,11,16
Advanced methods of cancer pain management, such as an implanted intrathecal drug delivery system (IDDS; i.e., SynchroMed), should be explored in certain circumstances. These circumstances include when systemic drugs fail to control moderate to severe pain; when adverse effects are intolerable; when neuropathic pain is present; when neuroablation has failed; in anticipation of symptom management; or upon patient request.9,11,16
In intrathecal (IT) drug delivery, a catheter is threaded into the intrathecal space by a pain specialist. A pump reservoir is surgically implanted in the body, typically in the abdomen, and connected to a catheter in the patient’s back.17 The reservoir size is 20 mL or 40 mL.11 Medication from the pump reservoir is delivered on a continuous basis into the intrathecal space, with the option of additional boluses upon patient request for breakthrough pain.9,11,18
Coverage for breakthrough pain is crucial because approximately two-thirds of patients experience it.12 With an IDDS, boluses can be given through a handheld remote-control device known as a personal therapy manager (PTM).18 Preprogrammed medication dosages, lockout periods and number of boluses allowed are entered by a pain specialist.18 Once delivered, pain relief is achieved in approximately 1 to 5 minutes.18 Setting adjustments secondary to pain level, functional status or adverse outcomes can only be performed by a provider through a telemetry programmer.11,19, 20
Intrathecal drug delivery bypasses the blood brain barrier, allowing medication to be delivered directly into the cerebrospinal fluid near the spinal dorsal horn.12,19,21,22 This direct effect on pain transmission pathways allows for equipotent drug delivery at a lesser dose than systemic medications – with decreased adverse effects.9,11,12,19,21,22 First-line medications for IT drug delivery and neuropathic pain treatment include hydromorphone (Dilaudid), morphine (MS Contin) and ziconotide (Prialt). Of these, morphine is the gold standard.9,11,23 Other options commonly used are opioids such as fentanyl (Sublimaze), sufentanil (Sufenta) and methadone (Dolophine); local anesthetics such as bupivacaine (Marcaine) and ropivacaine (Naropin); and adrenergic agonists such as clonidine (Catapres).
In some cases, a compounded drug with two or three medications is mixed by a pharmacist to optimize pain control through a multimodal approach on pain pathways.11 Preclinical and clinical research has found that medications within an IDDS remain stable and pharmacologically active for 90 to 120 days, depending on the particular medication.23
When treated with IT morphine, refractory cancer pain in general may decrease from 86% to 17% in patients after 8 weeks.23 A retrospective study of 292 cancer patients with IT drug delivery found a greater than 50% decrease in pain levels in 78.12% of subjects.22 An average pain score of 8.2 was reported at the beginning of the study, with a 2.6 median pain level noted at the end.22
A retrospective review was conducted on cancer patients who chose to initiate IDDS for pain management.18 The majority of these patients had colorectal and pancreatic cancer. Prior to IT drug therapy, 74.2% had uncontrolled pain and 65% classified the pain as severe.18 Four weeks post-IDDS, 3% reported severe pain, half had stopped all nonintrathecal medication, and pain scores decreased from an average of 6.5 to 3.1.18 Before IDDS, patients were administering a mean oral morphine equivalent of 796 mg/day for long-term pain control and 198 mg/day for breakthrough pain.18 After IT therapy, oral intake had decreased to 64 mg/day and 34 mg/day, respectively.18
A systematic review of four studies also demonstrated positive evidence about IDDS for cancer pain management. Statistically significant pain relief was noted in 61% to 80% of patients.21 One study reported that 100% of the patients had improved analgesia and enhanced mobility.21
In a randomized trial comparing IDDS with conventional medical management (CMM) for intractable cancer pain, 84.5% of patients with IT therapy reported adequate pain relief, compared to 70.8% of the CMM group. Researchers documented a 52% reduction in toxicity scores in the IDDS group versus a 17% reduction in the CMM group.24 In the IDDS group, fatigue and a decreased level of consciousness were significantly lower.24 This group had improved survival rates at 6 months.24
In addition to the statistical significance noted between pain levels, toxicity scores and quality of life when comparing IDDS with CMM, expert panel guidelines have reported that IDDS is less expensive than brand name oral regimens for cancer pain management.12 Oral generic medication management is the least expensive, but it is unlikely that cancer pain can be managed solely with generic medication. The equianalgesic dosing for intrathecal morphine when compared to oral morphine is approximately 300:1.22 Breakthrough medication use increases the cost of oral pharmacologic management substantially, making the PTM option associated with IDDS a cost-saving alternative.12
Potential Complications of IDDS
Complications can occur with IDDS, but compared to systemic side effects of CMM, they are less severe because a lesser drug amount is needed.9,11,12,19,22 A retrospective study by Lin et al reported a marked decrease in nausea, vomiting and constipation.19 Side effects observed are typically dose-related and can be divided into three categories: medication effects, surgical complications or mechanical complications. Reig and Abejon found an incidence of 16%, 8%, and 33% for mechanical, surgical and medication-related complications, respectively.22 Nausea, vomiting, urinary retention and pruritus are the most common side effects.11
Withdrawal and overdose from various causes may also occur with IDDS. Decreased body temperature, failure to refill the pump, catheter difficulties, low battery output, and radiation therapy in a site directly over the pump can attribute to withdrawal symptoms.11 Errors in medication administration or pump programming, increased body temperatures above 37 degrees Celsius, and low atmospheric pressure may lead to overdose with IT therapy.11
For patients who exhibit signs and symptoms of withdrawal or overdose, sensory or motor impairment, or changes in bowel or bladder function, the primary care provider should quickly ascertain drug type and potency, pump brand and a phone number for customer service for the pump’s manufacturer.20 The patient or family member should have a telemetry sheet with this information. Most patients require immediate referral to a pain specialist for further evaluation of potential complications that can be avoided with prompt treatment.11
Consultation
Pain centers can aid primary care providers with management of pharmacologic therapy and/or implementation of interventional techniques that may optimize pain management through action on various inhibitory pain pathways.9,16 Life expectancy of 3 months or longer is suggested to justify the initial cost of IT therapy.9,11,12,25 Considerations such as white blood cell count, platelet count, cancer site (e.g. metastasis to the epidural space), presence of spinal stenosis, infection or coagulopathies should be assessed prior to IDDS.12
Risks and benefits must be discussed with the patient, but no preexisting condition is an absolute contraindication for intrathecal drug therapy.12 IDDS does not inhibit ongoing cancer treatment or assessment.12,19
Patients with IDDS placement or revision require a 24-hour hospital stay to monitor for potential complications and assess efficacy of dosing.9,23 Patient teaching about the pump, PTM use and signs and symptoms of adverse effects is required before discharge. Regular follow-up at a pain clinic (every 60 to 90 days) should be scheduled for medication refills.19 At follow-up visits, therapy is assessed and any necessary pump adjustments are made.9,19 Battery life, which ranges from 4 to 7 years, is also assessed.11
Considering All Options
Research shows that current pharmacologic methods do not meet the needs of oncology patients and result in uncontrolled pain with a decreased quality of life.2,3,7,8,15 Interventional pain management is a relatively safe and effective option that should be considered early in the treatment, even prior to utilizing strong oral opioids.9,18,25 Palliative care techniques such as an IDDS can lead to increased patient satisfaction and ultimately a better quality of life for the patient.
References
1.Cain J M Hammes B J 1994 Ethics and pain management: Respecting patient wishes.Banner D J Qualitative interviewing: Preparation for pract Bercovitch M Waller A Adunsky A 2002 Multidimensional continuous pain assessment chart (mcpac) for terminal cancer patients: A preliminary report.Bercovitch M, et al. Multidimensional continuous pain assessment chart (mcpac) for terminal cancer patients: a preliminary report. Am J Hosp Palliat Care. 2002;19(6):419-425.
2. Torvik K Holen J Kaasa S Kirkevold O Holtan A Kongsgaard U Rustoen T 2008 Pain in elderly hospitalized cancer patients with bone metastases in Norway.Torvik K, et al. Pain in elderly hospitalized cancer patients with bone metastases in Norway. Int J Palliat Nurs. 2008;14(5):238-245.
3. Shvartzman P Friger M Shani A Barak F Yoram C Singer Y 2003 Pain control in ambulatory cancer patients-Can we do better?Shvartzman P, et al. Pain control in ambulatory cancer patients. Can we do better? J Pain Symptom Manage. 2003;26(2):716-722.
4. Tang S T Tang W R Liu T W Lin C P Chen J S 2010 What really matters in pain management for terminally ill cancer patients in Taiwan.Tang ST, et al. What really matters in pain management for terminally ill cancer patients in Taiwan. J Palliat Care. 2010;26(3):151-158.
5. McCance KL, et al. Biology, Clinical Manifestations, and Treatment of Cancer; Pain, Temperature Regulation, Sleep, and Sensory Function. In: Pathophysiology: The Biologic Basis for Disease in Adults and Children. Maryland Heights, MO: Mosby Elsevier; 2010: 360-395, 481-524.
6. Cain JM, Hammes BJ. Ethics and pain management: Respecting patient wishes. J Pain Symptom Manage. 1994;9(3):160-165.
7. Rogers M S Todd C 2010 Can cancer patients influence the pain agenda in oncology outpatient consultations?Rogers MS, Todd C. Can cancer patients influence the pain agenda in oncology outpatient consultations? J Pain Symptom Manage. 2010;39(2):268-282.
8. Williams J E Yen J T Parker G Chapman S Kandikattu S Barbachano Y 2010 Prevalence of pain in head and neck cancer outpatients.Williams JE, et al. Prevalence of pain in head and neck cancer outpatients. J Laryngol Otol. 2010;124(7):767-773.
9. Fitzgibbon D R Loeser J D 2010 Cancer pain: Assessment, diagnosis, and manaVissers KC, et al. Pain in patients with cancer. Pain Pract. 2011;11(5):453-475.
10. Fitzgibbon DR, Loeser JD. Forward: The Process of Pain In The Cancer Patient. In: Cancer Pain: Assessment, Diagnosis, and Management. Philadelphia, PA: Lippincott Williams and Wilkins; 2010: 17-23.
11. Bhatnagar S Mishra S Roshni S Gogia V Khanna S 2010 Neuropathic pain in cancer patients-Prevalence and management in a tertiary care anesthesia-run referral clinic based in urban India.Bhatnagar S, et al. Neuropathic pain in cancer patients–prevalence and management in a tertiary care anesthesia-run referral clinic based in urban India. J Palliat Med. 2010;13(7):819-824.
12. Ghafoor VL, et al. Intrathecal drug therapy for long-term pain management. Am J Health Syst Pharm. 2007;64(23):2447-2461.
13. Deer TR, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011;14:E283-E312.
14. Buttaro TM, et al. Management of Cancer Pain. In: Primary Care: A Collaborative Practice. St. Louis, MO: Mosby Elsevier; 2008: 1358-1364.
15. Raphael J, et al. Cancer pain: Part 1: Pathophysiology; oncological, pharmacological, and psychological treatments: A perspective from the British pain society endorsed by the UK association of palliative medicine and the Royal College of General Practitioners. Pain Med. 2010;11(5):742-764.
16. American Society of Anesthesiologists. Practice guidelines for cancer pain management: A report by the American Society of Anesthesiologists task force on pain management, cancer pain section. Anesthesiology. 1996;84(5):1243-1257.
17. Brogan S, Junkins S. Interventional therapies for the management of cancer pain. J Support Oncol. 2010;8(2):52-59.
18. Brogan SE, Winter NB. Patient-controlled intrathecal analgesia for the management of breakthrough cancer pain: a retrospective review and commentary. Pain Med. 2011;12(12):1758-1768.
19. Lin CP, et al. Efficacy of intrathecal drug delivery system for refractory cancer pain patients: A single tertiary medical center experience. J Formos Med Assoc. 2012;111(5):253-257.MccmMjklajklsjkldjklfjkslsjklaslkffdsdfsd
20. Medtronic. Synchromed II programmable infusion system: Clinical reference guide. 2012; 1-406.
21. Hayek SM, et al. Intrathecal therapy for cancer and non-cancer pain. Pain Physician. 2011;14(3):219-248.
22. Reig E, Abejon D. Continuous morphine infusion: A retrospective study of efficacy, safety, and demographic variables. Neuromodulation. 2009;12(2):122-129.
23. Deer T, et al. Polyanalgesic consensus conference 2007: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation. 2007;10(4):300-328.
24. Smith TJ, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040-4049.
25. Hawley P, et al. Intrathecal infusion for intractable cancer pain: A qualitative study of the impact on a case series of patients and caregivers. Pain Res Manag. 2009;14(5):371-379.